The FDA asks the court to stop producing Pfizer BLA documentation
Motion for summary judgement filed 10/17/2024
My readers are well aware of the FOIA lawsuit won by Aaron Siri in the U.S. District Court for the Northern District of Texas, which resulted in a court mandate to produce ~55K pages of Pfizer BLA documentation per month by the FDA. The suit was filed by Aaron Siri on behalf of the Public Health and Medical Professionals for Transparency (PHMPT) — a group of medical and public health professionals and scientists from Harvard, Yale, UCLA and other institutions who sued the FDA for “all data and information for the Pfizer Vaccine,” including safety and effectiveness data, adverse reaction reports, and a list of active and inactive ingredients.
The FDA produced these pages every month for about a year and a half. The productions ended in November 2023.
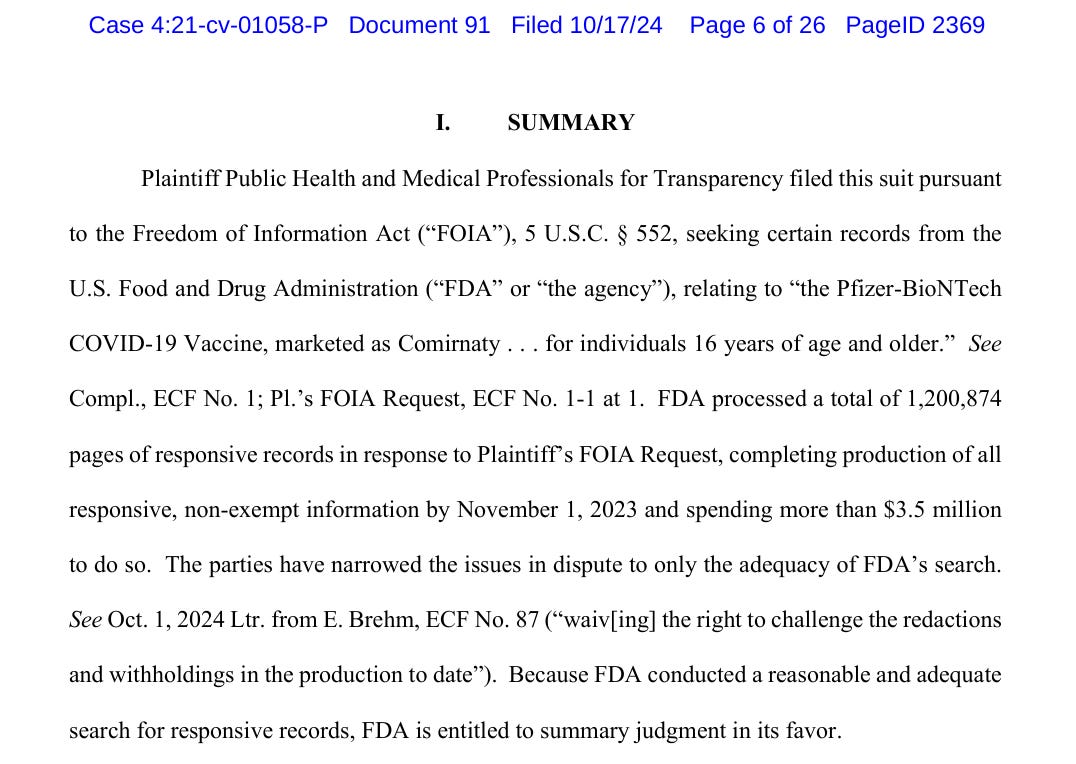
On October 17th, the FDA filed a motion for summary judgement:
The FDA is saying “we have produced 1.2 million pages of Pfizer documentation that was used to license Pfizer’s Comirnaty, and this constitutes everything we were mandated to do by the court”.
Here is the numerical page production summary:
They are asking to close this case now. In the court filing the FDA describes the elaborate process they went through, querying multiple internal databases and asserting that there is nothing left to produce.
I was asked to comment on this court filing by the Children’s Health Defense publication, The Defender. Here is the article by Suzanne Burdick.
Here is my quote for The Defender:
he documents released so far by the FDA are “inadequate” to assess the quality of Pfizer’s COVID-19 vaccine manufacturing process.
Of the more than 1 million pages produced by the FDA in response to the lawsuit, there should have been thousands of pages of manufacturing documents, she said. “Yet, almost none have been produced.”
Latypova said:
“There are several documents that became public after a leak from the European Medicines Agency in late 2020 that included Pfizer’s extensive communication with the FDA responding to queries on manufacturing process quality control.
“These documents have not been included in the FDA production in this lawsuit. The FDA should explain why.”
It’s important that the FDA release all manufacturing documents because the public deserves “assurances that the manufacturing process is high quality and rigorously controlled,” Latypova said.
Yet the FDA released “almost no documentation” from Module 3 of Pfizer’s Biologics License Application. Latypova explained:
“Module 3, or Chemistry Manufacturing Controls documentation, typically comprises approximately 20-30% of the new drug or biologics application as it deals with the manufacturer’s ability to demonstrate that their process ensures purity, potency and lack of contaminants in the final product as dispensed to the patient.
“Since there have been numerous reports, including in peer-reviewed publications, that Pfizer’s Comirnaty product is contaminated with non-conforming microRNA, DNA plasmids, SV40, different metals, protein, micro- and macro- objects in the vials — there are significant concerns with the manufacturing process failures.”
To provide more detail behind my statement above, I am going to summarize some relevant information which was previously published by me and others.
While the Pfizer papers were being produced on a monthly basis by the FDA, I collaborated with a group of researchers working with these files. There were several highly experienced data science people involved in the effort of processing the documents. It was a huge effort from numerous people working many hours, free of charge.
I published several in-depth analyses dealing with the scientific and regulatory fraud that was uncovered, specifically the review of non-clinical study package which showed that the product had several mechanisms of severe toxicity, and that it was damaging to the reproductive function. This review also showed that Pfizer, with complicity from the FDA, committed numerous scientific and regulatory fraud, passing irrelevant studies, omitting necessary studies, and using improper study designs to obfuscate potential for great harm:
Every month I inquired with my colleagues whether anything looking like Chemistry Manufacturing Controls (CMC) documentation was produced among these pages. No such materials have been included in the 1.2 million pages produced to date. The reason I was interested in that documentation is because many of us have also seen the package of CMC review materials that was leaked from the European Medicine’s Agency (EMA) in December 2020. I and several other colleagues published extensively on the woefully inadequate state of manufacturing at the time when the shots were deployed commercially world-wide. The senior staff at the EMA were stating in internal communications right around the initial commercial launch that “CMC issues” were going to be “the difficult bit”. Importantly, they are saying that the FDA told them this, i.e. the knowledge of the CMC problems originated at the FDA:
The CMC document, the so-called “Rolling Review” was also leaked and I and several other colleagues read it. It was a complete mess: the manufacturing process was incomplete, many parts of the submission dealing with critical quality control steps and analytical processes were missing entirely, or were incomplete, or were found outright fakes.
For example, Western blots were fake computer generated images, and the DNA plasmid map did not disclose the SV40 promoter, which was later found in every batch by Kevin McKernan and other colleagues.
As of late November, the EMA reviewers raised 140+ formal objections to Pfizer’s CMC submission, which still had many gaping holes and missing information. For reference 10-15 regulatory objections normally stop a pharmaceutical application from going forward until objections are resolved. Three Major Objections, i.e. formal red flags are discussed specifically in the emails below. I and others wrote extensively about MO#2 (lack of mRNA integrity). Here is an email from one of the reviewers, Evdokia Korakianiti and a response from Alexis Nolte discussing the problem and the impact (completely unknown and potentially very troubling) on the efficacy and safety of the product (this is when the now infamous Process 1 v Process 2 switch was identified):
It is evident from the responses to the concerns raised by Evdokia Korakianiti the EMA management waived arms and relied on “data that only FDA has seen”, but is “optimistic” and that FDA claimed mRNA breakage was a “theoretical concern”. Really?
Here are the emails indicating that the Major Objections were formally written up and subsequently disregarded by the EMA since the product was shipped commercially just a couple of weeks later.
The conditions stipulated by the EMA’s Conditional Marketing Approval were never fulfilled. Here is an excellent short summary of all the deficiencies that were known and disregarded at the time of product launch from
, Maria Gutchi:Maria’s latest article exposing Health Canada being caught with their pants down, or what Maria politely calls a “holy Toledo moment”. They realized that they were lied to in regard to SV40 in Pfizer shots and now need to cover it up by pretending this is all normal. Hilarious read if it weren’t for such a deadly subject matter:
Also from Maria’s article above, you can appreciate the finger pointing and email squabbles among FDA, EMA and Health Canada trying to both figure out and immediately cover up the fact that the FDA lied to all of them on behalf of Pfizer and helped cover up the nonexistence of compliant manufacturing and DNA with oncogenes in every vial.
I also have in my collection of documentation, several files of query resolution exchanges between the FDA and Pfizer, dealing with manufacturing process controls. These were found in the EMA leak. For example, this document - 54 pages of responses to the FDA’s questions about manufacturing quality controls and analytical procedures (I am including the Table of Contents here):
This means that the FDA was providing these communications to the EU regulators on regular basis, and that the FDA has these documents themselves. Yet none of these materials were produced in the 1.2M pages produced so far in the PHMPT lawsuit.
In conclusion, this shows that the FDA has in their possession numerous materials revealing their own knowledge that Comirnaty “as dispensed” is a poison (legally indistinguishable from poison due to no cGMP compliance and known presence of toxic materials), and that they lied not only to the US public about it, but to other global regulators (EMA and Canada). Albeit, I do believe the senior management at both EMA and Canada were complicit. Therefore, it is crucial in my opinion to continue this lawsuit and demand that the FDA produces the missing manufacturing process documentation among other materials that have not been produced yet.
For a one time donation:
Art for today: Garden Roses, oil on panel, 11x14 in.


















What this boils down to is that big pharma (fizer, most likely) is demanding their paid-off stooges at the FDA to tell the court to shove it. We ain't proving the other millions of pages of damning documents because we own the country and we can't be forced to give out the details of how we murdered millions with our poison mRNA garbage.
I agree Sasha, regarding complicity of senior officials in Canada (and world wide) in collusion with the miscreants at FDA. These folks are clearly criminals...traitors and murderers. Thanks for keeping the heat on...they are scared now, asking the courts for relief before the really important documents are revealed. Good job Sasha, and thank you.