Sarepta plot thickens...
I told you so 2 years ago: Senior officials at the FDA work to grow the market of severely ill and dying children.
I first published about Sarepta exactly 2 years ago, in July 2023. Briefly, Sarepta is a gene therapy company, and their drug was approved by Peter Marks (former head of CBER FDA) against recommendations of all FDA review teams who did not want to approve it. This was a highly unusual move and it sparked my interest. I am republishing the story below with added commentary on what has transpired since then. This story has recently come to a head, specifically:
On July 18, 2025 the FDA asked Sarepta to stop selling their drug, a gene therapy for Duchenne muscular dystrophy due to the deaths of 2 patients, and a very recent death of a clinical trial subject, all from liver failure. All 3 deaths are on Peter Marks’s hands, obviously.
Same day Sarepta issued a statement that they will not comply with the FDA request and will continue shipping their product to a slightly restricted group of patients (those who can still walk).
On Monday, July 21, however, Sarepta issued a statement saying that they agree to a temporary halt of shipping the drug would allow it time to respond to questions from the FDA and to complete an ongoing review of updated safety labeling for Elevidys.
Now, this is even more interesting than I thought 2 years ago. I have NEVER heard of such a case of defiance when it wasn’t about an EUA Countermeasure. Sarepta’s drug is not a military countermeasure and there isn’t a PREP Act declaration for Duchenne’s. Their drug, a gene therapy and not categorized a holy untouchable vaccine, is fully subject to the FDA’s regulatory authority, which gives them powers to send tanks and helicopters to every farm selling raw milk or hiding unvaccinated cows in the attic. All pharma companies know this, an none would dare to go against the FDA order to stop shipping the product. Let’s recall the history of this very interesting company.
Sarepta, in its own statement Friday night, said that its review of the Elevidys data shows no signs of new or changed safety signals. “We look forward to continued discussions and sharing of information with FDA in order to advance our shared purpose of protecting patient safety and informed access to care,” the company said.
“We will continue to ship Elevidys to the ambulant population,” Sarepta said.
Sarepta said it first heard of the regulator’s request earlier in the day through media reports, and only later received the government’s request. When the FDA seeks to pull a drug from the market, particularly for safety reasons, it often makes a voluntary request from companies.
But Friday night’s dueling statements will likely set up a showdown between the company and the FDA, whose recently appointed head of biologics, Vinay Prasad, has been highly critical of the gene therapy’s approval after it was pushed through by former FDA official Peter Marks. And Marks himself on Friday said he agreed with a pause in sales.
The agency also revoked the company’s platform technology designation that aims to accelerate the development of new therapies, citing the potential for “an adverse effect on safety.”
Perhaps the new FDA leadership doesn’t quite understand who owns Sarepta - explained in my article from 2023 with added commentary:
Continued from Part 1. Futile but hugely profitable is the nature of the scam with “orphan drugs” for “rare genetic conditions”. There is great interest in it from the highest ranks at the FDA.
Peter Marks, Director of CBER FDA, recently overrode reviewers’ call to reject Sarepta’s new Duchenne gene therapy.
The tiny clinical trial, designed to be a very low bar, failed.
The biotech’s exploratory analysis of the randomized trial suggested that the gene therapy had some beneficial effect in the younger boys, but didn’t make a significant impact in boys aged 6 to 7.
The FDA reviewers, i.e. those few remaining professionals at the FDA who still know something about drug development, balked, but Marks told them to shove it:
Three review teams — CBER Clinical Review, Clinical Pharmacology Review, and Statistical Review — did not recommend approval. Marks said he agreed “in large part” with the teams’ efficacy analyses that data from open-label studies and control groups outside Sarepta’s studies were not helpful. But he didn’t agree with their interpretation of very limited data in eight patients aged 4 and 5 from a randomized trial that Sarepta conducted.
Eight boys! At least it wasn’t 8 mice like with the DNA plasmid contaminated covid boosters. To be sure, Sarepta’s drug will be also contaminated with who knows what, but at least it is not yet required for your child to attend the school this fall.
For an FDA official to overrule the recommendation of their team is exceedingly unusual, but it has now happened twice for a Duchenne drug developed by Sarepta.
Lightning struck twice into the same spot - in 2016 Janet Woodcock pulled the same stunt for Sarepta approving their first drug:
In a highly controversial 2016 decision, Janet Woodcock, the director of CDER at the time, also overrode reviewers’ objections to approving Exondys 51, Sarepta’s first Duchenne drug, which is indicated for patients with a specific mutation. Another FDA official, Ellis Unger, disagreed with Woodcock’s decision and filed an appeal, sending the case to [Robert] Califf, [current FDA Commissioner] who sided with Woodcock.
Don’t you love Janet and Peter going out on the limb for such a little niche “orphan drug”? The profits are not going to be niche sized at all. The gene therapy, known as SRP-9001 or delandistrogene moxeparvovec, will be marketed as Elevidys. Elevidys will cost $3.2 million per patient, making it the second most expensive medicine in the US.
Is Sarepta Janet’s and Peter’s (and other HHS apparatchiks’) little business venture? You bet. While I can’t prove this, I can take an educated guess at several connections. One of the only 4 (unusually low number) investors in Sarepta is MidCap Financial, a mid-market lender based in Bethesda, MD, that also happens to have money management services, under the name Apollo Advisors (love the name!) In 2017, after Janet’s valiant effort on behalf of Sarepta, MidCap provided a $100M debt facility to Sarepta. Interestingly in 2018, they also provided a similar albeit smaller debt facility ($10M) to an obscure Ology Bioservices which has since metastasized into Resilience, a CIA and FDA linked biomanufacturing behemoth that also manufactures Moderna shots. Do you think Apollo Advisors manages money for the deep staters? Highly likely. Since this is debt financing (as opposed to equity investments), it is easier to hide government officials’ personal interest in the companies. As an added bonus, once the company stock goes up (because Janet rigged the drug approval for them) the returns to the equity holders are astronomical:
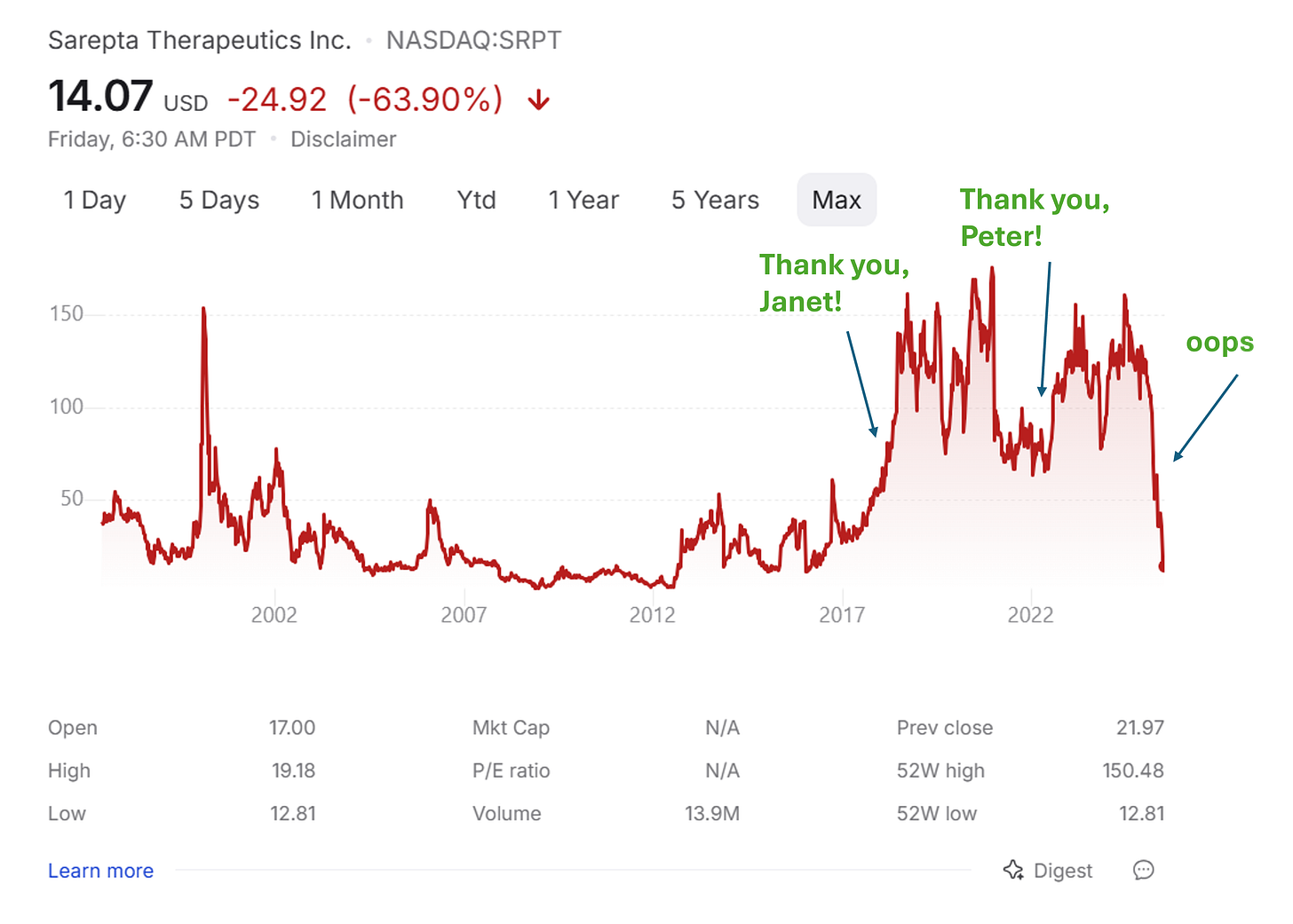
Updated stock chart as of July 2025:
Other connections between Sarepta and the US (and Israeli) government include current board member Kathryn Boor, who also concurrently serves on the United States-Israel Binational Agricultural Research and Development (BARD) Fund and the Science Board for the US Food and Drug Administration.
Another curious link is Dr. Nicaise (also Sarepta’s board member) who was a Senior Vice President of Strategic Development and Global Regulatory Affairs at Alexion Pharmaceuticals from 2008 to 2014.
Remember the company that had to write off $100M investment into Moderna after their joint gene therapy product failed because of safety problems? He was the guy at Alexion who made that deal or, at a minimum, signed off on it!
Just like Moderna, Sarepta has ties to Sweden and Karolinska Institute (and Swedish government as well). After I read the bios of Sarepta board members, the Moderna ones look like a roadside circus by comparison. Here is the real-real deal! Another board member at Sarepta is a Swedish luminary of simply incredible wattage. Behold Dr. Wigzell whose academic career includes serving as Chairman of the Nobel Prize committee and the Karolinska Institute.
‘nuff said.
In 2023 I wrote:
If you think Sarepta/Peter/Janet are going to be satisfied with charging $3 million per each 4-5yo boy with muscular dystrophy, you need to expand your horizon. They will rapidly grow the market. The covid injection poisons will deliver a variety of “rare genetic conditions” among currently injected and in children born to vaxxed parents.
I was right about that! Notice that the Peter-Marks-approval was for boys aged 4-5, based on 8 subjects (!) cherry-picked data from the study that somehow “convinced” Peter that the $3M/dose drug should go on the market. Now, what’s also interesting - the patients that died were not 4-5 yo, they were teenagers! So the company was already selling the product to unapproved population, because of course. That didn’t bother anyone, until the patients started dying from liver failure.
If that is not enough, their real targets are…
“Transgender” (mentally and physically abused) kids of course! I am placing this bet now, and please check with me in a couple of years, or maybe sooner. you heard it here first. Muscular dystrophy can be induced by using the chemical castration drug Lupron which is a staple of every child mutilation “clinic” today.
The Bone Journal reported that puberty suppression resulted in decreased bone growth in adolescents with gender dysphoria.
The reported side effects of Lupron are staggering both in the breadth of physiological systems affected and the depth of symptom severity experienced (a partial list). Indeed, everything from the brain and nervous system to the musculature, skeletal, gastrointestinal and cardiac systems are affected by Lupron, sometimes irreversibly. This is in addition to thyroid, gallbladder and pancreatic side effects.
As you can see, Lupron is a wonder-drug by which symptoms of “rare genetic conditions” can be replicated in kids that were born without them, and then make a fortune, including for the children mutilation “clinics” by selling gene therapies for these conditions at $3 million/pop.
Additionally, the mass injections with mRNA/plasmidDNA/toxic metals in DARPA hydrogel will bear lots of fruit and produce more “rare genetic conditions” to finish off profitably, just like what Sarepta is doing.
Back to current day news. Marty Makary went on The American Thought Leaders with Jan Jekielek, where he lied about many things, most egregiously about mRNA shots, for over an hour. This bit stood out to me:
Makary acknowledges that covid vaccines killed and injured people, but refuses to stop the shots or force recall, citing the need for “proper science”:
MAKARY: “I personally know of people who have been injured by the vaccine. I personally know of friends who have lost a loved one from the mRNA COVID vaccine … People have a right to be angry. They have been deceived … I would ask people to be patient with us as we do this the proper scientific way.”
Here is my advice to Sarepta, or any company or farm that the FDA directs to recall their product: respond to the FDA by saying “you have the right to be angry, Marty. But we ask you to be patient with us and do this the proper scientific way”. Then proceed to ship your product. Clearly, the old FDA and the new FDA are the same FDA, and due to continuously lying and covering up the mass murder that the old FDA enabled, the new FDA has obliterated any shreds of credibility in their regulatory authority.
Art for today: Chicken friends, watercolor, 9x12 in.






The most abundant element in the universe is not Hydrogen; it's evil.
MAKARY: “I personally know of people who have been injured by the vaccine. I personally know of friends who have *lost a loved one* from the mRNA COVID vaccine … People have a right to be angry. They have been deceived … I would ask people to be patient with us as we do this the proper scientific way.” [Asterisks mine]
Be patient? Proper scientific way? How about proper LAWFUL way, ya asshole? What does "lost a loved one mean"? It means they are now DEAD and were murdered, asshole. Fuck!