Genetic Veterinary Vaccines
Updated full report with bonus for paid subscribers - downloadable highlighted USDA listing of veterinary vaccines (at the end of post, scroll past the artwork)
Vaccines based on synthetic nucleic acid materials (DNA/RNA) are already used in animal vaccinations. Most of these products are either recombinant protein or viral vector-based.
General Position – all vaccines derived from plasmid DNA as raw material have DNA plasmids as intended or unremoved “contaminant” and thus have potential to transfect cells, damage cellular processes and genomes and/or microbiomes of both target and off-target species. Depending on how many cells are affected and of what type, this damage may be irreversible and may affect reproduction and offspring in a variety of ways (all of them bad).
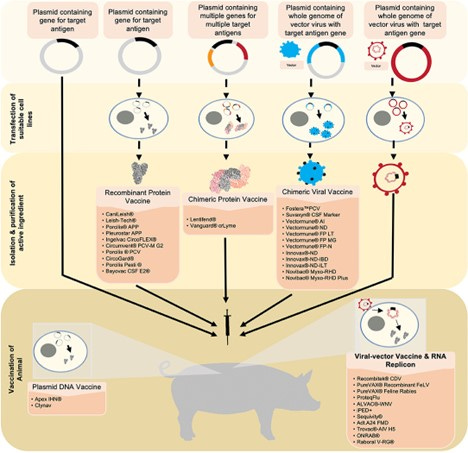
Figure 1.
Source: Scientific review of “next generation” genetic-based vaccines in development or on market.
There are 6 “next generation” genetic-based vaccines in development or on market (see Fig 1). The categories include:
1. Plasmid DNA vaccines
2. Recombinant protein vaccines
3. Chimeric protein vaccines
4. Chimeric viral vaccines
5. Viral-vector vaccines
6. RNA Replicon vaccines
Note that all these categories of vaccines use DNA plasmids as a starting point of manufacture, most of them - as raw material to be transformed to other types of biological substance, except the 1st category where plasmid DNA as an injectable substance is the final product.
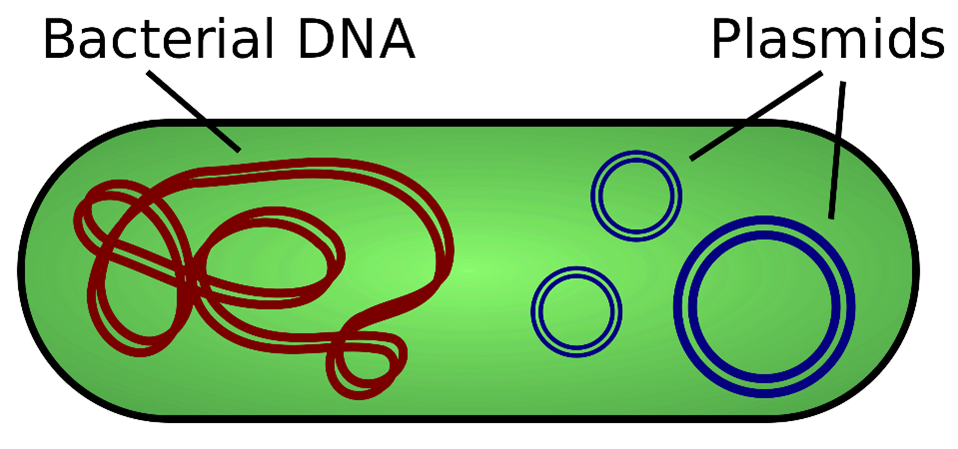
Figure 2.
Remember this fact: all raw materials and all in-process chemicals used in biomanufacturing end up in the final product. It is impossible to produce any “biologic” precisely and purely to spec, and the FDA has largely removed regulations and oversight from these factories.
DNA in plasmids are circular pieces of synthetic nucleic acid code (NOT natural DNA, it’s a chemical molecule) that is introduced into the vats of e.coli cells which naturally pick up plasmids and replicate quickly. This is the way DNA is grown at scale in biomanufacturing. After DNA is grown, it is harvested by an antibiotic wash (to kill the e.coli). Thus, synthetic DNA code in plasmids always contains genes for antibiotic resistance. This alone poses serious issues related to increasing resistance to antibiotics, and it will be magnified with increased use of these products in both human and animal products.
DNA plasmids are considered a “transfectant” – a substance designed to transfer “genetic” code into cells and nuclei of the target host from another organism or species. I am saying “genetic” in quotation marks, because the code that is being artificially created and transferred is only genetic in a sense that it will wreck your genetic processes in those cells that it successfully breaks into. It is a synthetic chemical molecule encapsulated in other chemicals and has nothing to do with normal genetics of living organisms.
Categories 2-5 are based on cell lines transfected with plasmid DNA carrying “gene of interest”, sometimes by itself, sometimes a “vector” is involved such as a chemical entity that is claimed to be a “replication defective virus”. I can’t address decades of fake science in one article, so where you see quotes - I am only stating what these things are claimed to be in fake science. There is no real validity to this nomenclature.
Category 6 is the most analogous to the human covid-19 shots – it is based on RNA encapsulated nanoparticle. Its starting raw material is still DNA plasmids which are supposed to be translated into RNA by a chemical reaction, and subsequently completely removed.
We know the removal of plasmid DNA does not really happen. My current understanding of the manufacturing process leads me to believe that it is not possible to remove DNA plasmids cost-effectively and without destroying the product. The human covid shots are always found “contaminated” with plasmid DNA (up to 30% of the vial volume in independent lab tests so far). Kevin McKernan’s work is available at
albeit his writing is highly technical:
DNA plasmids persist in the injected host, and it is not clear for how long. In most literature it is claimed this is “transient”. However, in this study from Italy DNA plasmids were detected in fish 320 days in fish after vaccination:
According to the EU regulatory definitions, BOTH vaccine and its recipients could become GMO, if the product is designed for genetic/biome integration… As discussed by Collins et al, “DNA Vaccines in Fish and Aquaculture.”
What happens if the product was technically not designed for genome integration, but somebody “forgot” to remove the genome integrating DNA plasmids from it or didn’t remove them to prescribed limits, and also “forgot” SV40 promoter in the plasmids, and then “forgot” some other undisclosed components that just all completely accidentally and coincidentally happen to comprise a genome integrating machinery?
How would you know if the substance your animals are being injected with contains those “contaminants”? How would a veterinarian, or a farmer know/be able to detect this? They do not have access to the labs/equipment/expertise to find these dangerous “contaminants”. Given FDA’s total lack of interest in regulating biomanufacturing facilities, the only way to address this problem is to not use the product at all.
The potential for contamination or intentional non-removal of plasmid DNA from all these categories of vaccines is accompanied by deep indifference of the regulators to this issue. In fact since 2019, the specific duties of the FDA inspectors, including independently testing contents of the produced products have been eliminated by the FDA rule change. Therefore, my overall recommendation is to treat ALL “next generation” vaccines as a potentially gene- and/or microbiome damaging technology which carries excessive risks of damage to not only livestock but also other animals or humans either accidentally exposed or working with the animals, or processing or consuming the food products derived from them.
How to tell whether a particular animal vaccine is potentially derived from DNA plasmids?
USDA Nomenclature.
USDA uses “True Names” for biologic vaccines. The True Name includes all of the antigenic fractions of the product for which there is a label claim. (Thus, the product may contain other “unclaimed” antigens.) In regard to the manufacturing technology, the following conventions apply:
“Modifiers of the product form may be used at the end of the list of antigens and are separated from the antigens by commas (e.g., Killed Virus, Avirulent Live Culture)”.
USDA also states that there is a lot of confusion about naming new generations of recombinant vaccines and other DNA and RNA related technologies. I propose simple heuristics for identifying them based on USDA information or promotional material from manufacturers:
The following words indicate that the product might be a gene- or microbiome- altering, either by design or by potential contaminants:
RNA, DNA, plasmid DNA, replicon, platform, gene of interest (GOI), sequence or sequence version, viral vector, chimeric/chimera, recombinant, protein.
If the product is for “emergency use” or designated as a “countermeasure.”
How to read product codes:
Look at the Fifth digit in the product code, which is typically in the format XXXX.XX
If the fifth digit is P, D or R – these products potentially contain genetic codes (nucleic sequences), most of them modified, and they have a high potential to alter genomes and microbiomes. The most concerning are the products coded P and D. R-coded ones, especially approved in recent years, are highly suspect in my opinion, but may or may not be as dangerous. All of them have high potential for contamination with plasmid DNA which would make them highly problematic.
In priority use/order, if more than one designation applies:
U=unlicensed (DEREA)
P=Platform conditional product
D=nucleic acid vaccine
R=contains recombinant seed(s)
B=ballistic product
I=implant product
C= licensed for use only in the State of California due to California Assembly Bill No. 1709 approved by the Governor September 23, 2010, and filed with the California Secretary of State on September 24, 2010
In reading the short product descriptions, you may run into the following terms in Table 2, whose definitions have been outlined below. It is important to note that even if the short product description does not explain the technology if the product is in the D or R categories, the product is almost guaranteed to involve plasmid DNA in the manufacturing process. Products in category ‘P’ are similar to the COVID-19 vaccine technology and, as a result, are likely also suspect.
Bonus for paid subscribers: For a downloadable USDA veterinary vaccine listing highlighted (red) with products that are highly likely derived from DNA plasmids, scroll to the end of the post, after the artwork.
Previous articles on this topic:
For a one-time donation:
Art for today: Bridges, watercolor on paper, available as a fine art print.
Downloadable USDA listing which identifies veterinary vaccines produced from DNA plasmids:
Keep reading with a 7-day free trial
Subscribe to Due Diligence and Art to keep reading this post and get 7 days of free access to the full post archives.